Bioluminescence in Lanternsharks and its Impact on Communication via Identification
By Braden Furness '20
BIOL-320: Evolution
This assignment asks students to think like research evolutionary biologists. It consists of finding a gap in knowledge and developing a research proposal that would help fill in that gap. Students can choose their topic. They need to do extensive bibliographical research, develop a hypothesis, and a way to test it through experiments or observations. Braden merges his interest in sharks, bioluminescence, and sexual selection to explore how evolution has resulted in incredible forms of life.
-Paulina Mena
Bioluminescence is a key part to many organisms and their lifestyle, both on land and in the ocean. From fungi to fireflies and microbes to deep water fish, this advantageous chemical process seems to touch every living thing in one way or another. Its evolutionary history is very much still a mystery though, with the sheer number of taxa that display this phenomenon. It is estimated that at least 40 to 50 independent evolutionary events have occurred among taxa to produce bioluminescence (Haddock, 2009). This comprises of representatives from most phyla including Bacteria, Pyrrophyta, Protozoa, Porifera, Cnidaria, Ctenophora, Rhyncocoela, Nematoda, Mollusca, Annelida, Arthropoda, Bryozoa, Echinodermata, and Chordata. However, true plants and higher vertebrates such as amphibians, reptiles, birds and mammals are an exception to this trend (Bjorn, 2008). This leads to a wide variety of functions for bioluminescence which has been dissected into five main roles for organisms: reproduction, defense and camouflage, food procurement, protection from reactive oxygen species, and DNA repair via activation of repair enzymes (Bjorn, 2008). Therefore, there is no one way to produce light, with many chemical mechanisms documented in the animal kingdom. Some similarities among these bioluminescent mechanisms include the use of an enzyme called luciferase which catalyzes some sort of peroxide and uses oxygen in the process. This reaction then produces a pigment which, depending on the organism, can directly produce light, or transfer it to another emitter (Hastings, 2014). A large difference is seen in the kinds of luciferase present in each organism as documented in Figure 1 which arises from the large amount of independent evolutionary events. However, much is still unknown about the bioluminescent process that occurs in deep sea fish, especially sharks.
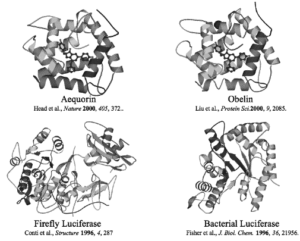
Figure 1: Displayed are 4 different luciferases, the top two from coelenterate, bottom right from a firefly and the bottom left from a bacterium (Hastings).
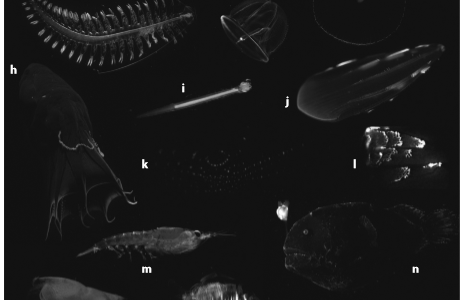
At about 200 meters below the surface, light from the sun quickly drops off with visibility minimal. At this depth, and deeper, fish and other marine organisms begin to show traits of bioluminescence such as anglerfish, sea stars, and plankton. Figure 2 gives several more examples and displays the colors that are also produced by some of these deep-sea dwellers. This makes bioluminescence a key evolutionary advantage in this habitat. Deep-water organisms utilize light producing organs called photophores to generate and produce their own light. Generally, emitting light is measured through nervous control, but fish also contain secondary means such as physiologically changing parameters of the bacteria, or utilizing dark shutters known as chromatophores (Claes, 2010). This heightened control over their bioluminescence allows for it to be used in a wide variety of behaviors. This includes defensive measures such as counterillumination, startling predators, misdirection or warning coloration. Offensive uses include luring prey, stun, or illumination. Another separate behavior that has been documented is mating identification between individuals (Haddock, 2009).

Figure 2 (Haddock, 2009).
Sharks are not an exception to bioluminescent use in deep water. Of the 440 plus known species, two shark families, Etmopteridae, and Dalatiidae, exhibit bioluminescence. In Etmopteridae, there are five genera: Etmopterus, Aculeola, Centroscyllium, Miroscyllium, and Trigonognathus while Dalatiidae features seven genera: Dalatias, Euprotomicroides, Euproto-micrus, Heteroscymnoides, Isistius, Mollisquama, and Squaliolus (Compagno, 2005). Combined, these group of sharks feature over 50 individual species making it one of the largest and most diverse groups of sharks. In lanternsharks, Etmopterus, light is produced through photophores located on its belly and flanks, similar to that of bioluminescent fish. However, hormones control their photophores, unlike other fish systems. Prolactin and melatonin are found to be the main contributors to this process, acting as triggers while alpha-MHS inhibits (Claes, 2010). This process is conducted through photocytes, located in the photophores, which are lens like structures that specialize in producing light (Claes, Mallefet, 2015). This allows for lanternsharks to possess great control over their bioluminescence for extended periods of time.
All 37 lanternshark species display slight differences in patterns and organization of these photophores. Some species like the cylindrical lanternshark, E. cateri, lack any concentrated photophores while other sharks such as the velvet belly, E. spinax, feature photophores on both their belly and flanks (Compagno, 2005). A similarity between all lanternshark species consists of photophore concentration on their bellies. This indicates counterillumination as one of their main functions. Even 200 meters below the surface, some light is able to seep down, giving just enough illumination to cast a shadow on predators or prey below. Utilizing the glow from photophores, lanternsharks can mask their shadow and match the sunlight filtering above. A similar process occurs for predators and prey looking down into the depths. Lanternsharks’s lack of photophores on their dorsal side and dark colored bodies, then help them blend into the black water that stretches below them. However, lanternsharks do feature lateral photophores which sets them apart from other bioluminescent sharks. These lateral patterns do not offer any assistance in counterillumination which leads to the fact that they must have a different purpose.
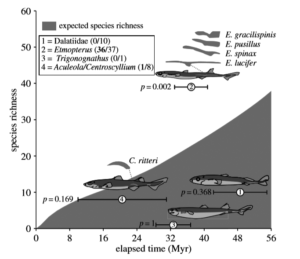
Figure 3: The numbers in parentheses indicate the ratio of species with flank markings versus total number of species (Nilsson, 2015).
The similar belly and lateral photophore patterns have proven difficult to scientists to identify and classify lanternsharks without in-depth analysis of each and every morphological mark (Coelho, 2008). Speciation, thus, is very predominant in this genus, which is unusual as most other shark genera who also feature lateral markings have very little diversity (Nilsson, 2015). Lanternsharks are also comparatively a younger clade which would indicate less diversity as well. In Figure 3, looking at a 95% confidence interval of expected species richness of bioluminescent sharks, lanternsharks fall well outside the interval. This means the null hypothesis that speciation is occurring at a normal rate is rejected. Rather, some event, or process is speeding up this development. This amount of speciation is unique and gives insight as to what difference lanternsharks have, as compared to the rest of their family Etmopteridae and the other bioluminescent family Dalatiidae. In this case their lateral photophores have come under scrutiny as the factor that changes this process. From a predator, prey aspect, they are completely useless for counter-illumination, which may indicate an evolutionary event in the past that made this trait beneficial for a different reason. It has been recently suggested that these lateral photophores may promote sympatric speciation through reproductive isolation (Nilsson, 2015). The proposed question now is: does this suggest and draw the conclusion that lanternsharks are using their bioluminescence to identify and communicate with individuals to find a mate?
In this investigation, bioluminescence in lateral photophores on lanternsharks, is tested to demonstrate the impact it has on mating via communication with other individuals. This includes locating and attracting a mate, as well as distinguishing between different species in the darkness of its habitat. The slendertail lanternshark, E. molleri, is tested on to generalize Etmopterus in this study due to its use in prior studies as a comparative measure and known morphological differences between male and female. The giant lanternshark, E. baxteri, and blackbelly lanternshark, E. lucifer, are used as interactive species with the slendertail due to their similar habitat and similar lateral photophores.
Materials and Methods:
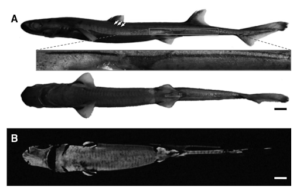
Figure 4: A is the lateral and ventral profile of a slendertail done in normal light, displaying the lateral photophore in the red box that is similar to shape and size seen on giants and blackbellies. B shows ventral side with luminescence (Claes, Mallefet, 2015).
Thirty slendertail lanternsharks, ten giant lanternsharks, and ten blackbelly lanternsharks were caught via deep water hook and lines off the east coast of New Zealand near Portland Island, along the Hikurangi Trench at depths about 400 meters. 14 slendertail males measured in length from 39-42cm, 16 slendertail females measured in length from 40-46cm, seven giant females were measured between 66-69cm, three giant males were measured between 54-57cm, five blackbelly females were measured between 32-37cm and five blackbelly males were measured between 27-29cm. They were then transported via cold, oxygenated seawater bags to the Okinawa Churaumi Aquarium where individuals were then placed in separate tanks in a dark room with seawater averaging 5-10 degrees Celsius. Pictures were then taken of each individual’s ventral and lateral sides in normal light and in darkness to identify photophore locations and patterns. These patterns were then measured and compared between each individual of the separate species, giving means of individual photophore zones for the slendertail, giant, and blackbelly lanternshark. Figure 4 displays a sample photograph of a slendertail that was used as a guide for acquired pictures. Utilizing the photographs and mean measurements of the photophore zones, male and female models of each species was made with anatomically correct body part ratios. The models were made with neutrally buoyant foam with a weight in the middle while the photophores were made of faint OLED lights imbedded beneath the foam, allowing control over turning them off and on, as well as brightness via electrical currents.
One at a time, models of the opposite sex of the live slendertail lanternshark were suspended in its dark tank. Each model conducted 47three trials on the live individual, showing different behaviors each time. The first model would show no interest in the live slendertail individual, the second model would show behavior of curiosity with the live slendertail while the third model would display behavior related to being in heat/wanting to mate. This was done with the slendertail, giant, and blackbelly models for a total of nine trials for each live slendertail for both males and females. The interactions witnessed between the model and live individual were then recorded using time in seconds as a form of measurement and comparison, with a large focus on physical observations such as use of photophores.
Expected Results:
The expected results of the experiment include extended amounts of interactions between the live slendertail and the slendertail model while limited interactions occurred between the live slendertail and models of the giant and blackbelly. This would indicate that lanternsharks have developed the ability to identify and distinguish their particular species from other lanternsharks in low visibility. With the lateral photophores isolated as the only known form of communication the sharks could exhibit in this experiment; a correlation would be safely assumed in this case. Therefore, a conclusion could be drawn due to variations in lateral photophore patterns seen in the giant and blackbelly lanternsharks compared to the slendertail lanternshark. Consequently, these expected results might be able to help explain the high amount of speciation occurring within the genus and how mates are able to locate one another in the dark depths of the ocean.
Works Cited
Haddock, Steven H.D., Moline, Mark A., Case, James F. (October 2009). Bioluminescence in the Sea. Annual Reviews Marine Science, 443-493.
Bjorn, Lars Olof. (2008). Photobiology. Spring Street, New York: Springer.
Hastings, J. Woodland (August 2014). Dinoflagellate Bioluminescence and its Circadian Regulation. http://photobiology.info/Hastings.html
Claes, J. M. (2010). The lantern shark’s light switch: turning shallow water crypsis into midwater camouflage. Biol. Lett., 685-687. Compagno, L., Dando, M., & Fowler, S. (2005). Sharks of the World. Princeton, New Jersey: Princeton University Press.
Claes, J. M., & Mallefet, J. (June 2015). Comparative control of luminescence in sharks: New insights from the slendertail lanternshark. Journal of Experimental Marine Biology and Ecology,87-94. Retrieved September 26, 2018, from www.elsevier.com/locate/jembe.
Coelho, Rui, Erzini, Karim. (2008). Identification of deep water lantern sharks (Chondrichthyes: Etmopteridae) using morphometric data and multivariate analysis. Journal of the Marine Biological Assoc. of the United Kingdom, 199-204.
Claes, J. M., Nilsson, D-E, Mallefet, J., Straube, N., (2015). The presence of lateral photophores correlates with increased speciation in deep-sea bioluminescent sharks. R. Soc. Open sci. 2:150219. http://dx.doi.org/10.1098/rsos.150219