
Birth Control: From Lab to Market
By Katie Wang
Chem 425: Advanced Topics in Biochemistry: Medicinal Chemistry
Katie is majoring in Biochemistry and Spanish. She wrote this last spring as part of our Advanced Topics in Biochemistry: Medicinal Chemistry. For this assignment, students were tasked with writing an 8-10 page literature review on a contemporary topic related to medicinal chemistry using the Journal of Medicinal Chemistry as a template. They were given some leeway on how to cover the topic (as a review), but it needed to include some context, synthetic components, pharmacokinetics and pharmacodynamics.
-James Shriver
I. Introduction
Between the years 2015 and 2017, a national survey conducted in the United States found that 64.9% of 72.2 million women used contraception. Of those women, the second most utilized form of contraception was an oral contraceptive pill. The only form of birth control which exceeded the use of the pill was female sterilization.1 Based on this data, it was clear that when it came to reversible contraceptives, the pill was the form most heavily depended on. Although birth control is now considered an “essential medicine,” the need for the pill was not always recognized nor prioritized.2
One woman in particular pushed for the development of the birth control pill. She felt women should have control of their bodies, especially when it came to pregnancy. This woman, Margaret Sanger, was a reproductive rights activist who founded the American Birth Control League–now known as the Planned Parenthood Federation.3 Upon meeting biologist Gregory Pincus in 1950, she asked him to develop a cheap, dependable and reversible form of oral contraception. Nine years later, the first birth control pill was approved by the FDA, fundamentally changing the available methods of reversible contraception.4 5
II. Pharmacodynamics
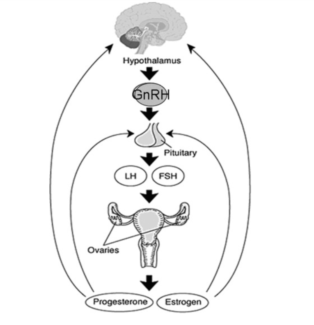
Figure I. The female reproductive hormone cycle.8
In the female reproductive system, various hormones are at work to stimulate ovulation. First, the hypothalamus releases gonadotropin releasing hormone (GnRH) to the anterior pituitary gland located in the brain. When this occurs, the anterior pituitary is stimulated to release the follicle-stimulating hormone (FSH) and the luteinizing hormone (LH).6 Both hormones target the ovaries and stimulate ovulation. When a woman is not ovulating, her ovaries are releasing estrogen and progesterone. These hormones act to prepare the body for and to maintain pregnancy.7 Additionally, these hormones travel through the blood to the hypothalamus and anterior pituitary. When progesterone and estrogen reach these glands, they inhibit the release of GnRH, LH and FSH so ovulation cannot occur. If the levels of progesterone and estrogen drop, the hypothalamus and anterior pituitary are then allowed to release the necessary hormones to stimulate ovulation.6 A diagram of the cycle can be found in Figure I.
When a woman begins to take birth control, she is introducing synthetic forms of progesterone and estrogen into her system. In the same way that natural progesterone and estrogen work, these synthetic hormones target the hypothalamus and anterior pituitary to prevent the release of GnRH, LH and FSH.6 Essentially, the brain begins to believe that the body is pregnant and ovulation is inhibited.3
III. Synthesizing Progesterone
Russell Marker
One of the key ingredients in birth control is the steroid hormone progesterone. Prior to 1944, progesterone was extracted from animals in low concentrations and required a long purification process before it could be used. Due to these complications, the cost of one gram of the hormone was around $200. To find more potent forms of progesterone, Russell Marker turned to plants and discovered the steroid diosgenin. Although structurally different to progesterone, the chemist was able to convert diosgenin into progesterone with a 60% yield.3 The synthesis can be found in Figure II.
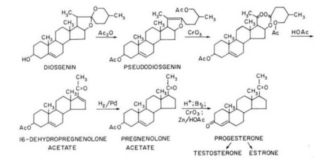
Figure II. Synthesis of progesterone from diosgenin.9
Once Marker determined progesterone could be synthesized from plant steroids, he searched for plants with more abundant sources of diosgenin. Most plants contained little to no diosgenin, except for a wild yam found in Mexico. With a source and procedure, the price of progesterone dropped to $80 a gram. In the years to come, concurrent with the finding of a Mexican yam with roots ten times richer in diosgenin, the price of progesterone was cut down to $1.75 a gram.3 10 Upon these discoveries, the active ingredient in birth control was both easily accessible and reasonably priced.
Gregory Pincus
In the 1930s, several animal experiments indicated that ovulation could be inhibited with daily injections of progesterone. Following this work, Gregory Pincus and his associate Min- Chueh Chang conducted experiments on rabbits and rats, utilizing progesterone as a form of contraception. Quickly, they were able to develop a hormone regimen which prevented pregnancy.3 At around the same time, a professor named John Rock was trying to use progesterone and estrogen as a method of curing infertility. Since both hormones are present during pregnancy, Rock’s idea was to produce a pseudo state of pregnancy in an attempt to make patients more susceptible to pregnancy.7 During the trial, 80 infertile women received hormone treatments; after the trial, 14 women became pregnant. Interestingly, none of the participants were able to become pregnant during the trial.3
After meeting in 1951, Pincus and Rock began working together to produce the perfect combination of hormone to administer to patients. One idea was to completely remove estrogen and simply inject progesterone into patients. Although this method proved effective, patients experienced unpleasant side effects such as breakthrough bleeding (bleeding or spotting experienced between normal menstrual periods). By reincorporating estrogen, even in a small dose, bleeding from the uterus was inhibited.7 Upon this discovery, Pincus and Rock had the necessary information to inhibit ovulation and prevent pregnancy. Despite having this knowledge, there was one major setback. The only way to administer the necessary hormones to prevent pregnancy was through injection. Although possible, this method was not preferable. Instead, they needed an orally active form of the hormone progesterone.4
Carl Djerassi
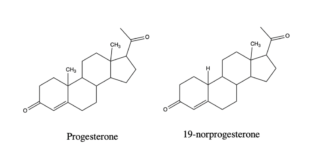
Figure III. Structure of progesterone and 19-norprogesterone.
When synthetically made from plants, progesterone must be given via an injection. If ingested orally, the hormone is either destroyed by stomach acid and enzymes, or it gets stuck in the intestines and cannot reach the blood.6 10 Original attempts to chemically alter the structure of progesterone so it could be taken orally, diminished the biological activity of the hormone.11 In 1951, a chemist named Carl Djerassi was able to synthesize the first orally active progesterone: 19-nor- 17ɑ-ethynyltestosterone, or norethindrone.
In the early 1940s, the chemist Maximilian Ehrenstein published an article describing how an impure form of 19-norprogesterone had an increase in biological activity when tested in rabbits.10 11 Inspired by the work, Djerassi began focusing on replacing the methyl group at the 19 position with hydrogen. A modified Birch reduction resulted in the desired product, 19-norprogesterone (Figure III).12 When tested biologically in rabbits, the modified hormone proved to be 4-8 times as active as the natural hormone.11 Although the hormone could be modified to increase activity, it was still not orally active.
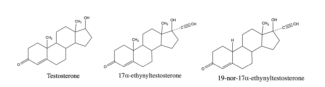
Figure IV. Structure of testosterone, 17ɑ-ethynyltestosterone
and 19-nor-17ɑ-ethynyltestosterone.
In 1938, a chemistry group at Schering A.G. in Berlin demonstrated that the addition of an acetylene group at position 17 of the sex hormone testosterone not only made the hormone orally active but also changed its biological activity. The hormone now had low progestational activity.10 11 Combining the two methods of adding an acetylene group and removing a methyl group from testosterone, Djerassi was able to synthesize 19-nor-17ɑ-ethynyltestosterone, or norethindrone, an orally active form of progesterone (Figure IV).
Further Developments
Over time, several other orally active progesterones have been developed. The original pill contained the compounds norethindrone and norethynodrel. Although effective, these synthesized hormones were not selective and could interact with other androgen receptors, resulting in unwanted side effects.6 To increase selectivity for progesterone receptors, so as to reduce side effects, other synthetic progesterones like levonorgestrel, desogestrel and norgestimate were developed.2 Now, there are about ten different synthetically made progesterones found in various birth control pills, as well as two different forms of estrogen.6 Although structurally different, all forms of progesterone act in the same way so as to inhibit ovulation as a way of preventing pregnancy.
IV. Finalizing Steps
Animal Trials
In the 1930s, rats and rabbits were injected with various combinations of hormones. Based on the results of these trials, it was determined that high levels of estrogen could prevent pregnancy.3 13 Pincus himself began animal trials to further understand the necessary hormone concentrations needed to inhibit ovulation. Based on his results, it was progesterone, not estrogen, that was necessary to prevent pregnancy. Once Pincus had produced a hormone regimen which worked well for rabbits and rats, he was ready to test his method on humans.13
Clinical Trials
Original human trials were conducted by Rock who was focused on curing infertility. By suggestion of Pincus, Rock varied hormone concentration and administration.3 When Rock’s infertility trials were over, the pair began clinical trials on a larger scale with 60 patients from Free Hospital. While none of the patients became pregnant during the trial, over half of the participants chose to withdraw due to unpleasant side effects.13 These side effects included breast tenderness, weight gain, increased blood pressure and mood swings, all of which are also indicative of pregnancy.4 Frustrated with their inability to keep patients in the trial, Pincus and Rock turned to women in a Massachusetts asylum, Worcester State Hospital. While working there, they started hormone therapy on 16 patients. Although none of the patients dropped out, they experienced similar unpleasant side effects as the women in the original trial. In 1955, Pincus and Rock began to branch out and started clinical trials in Puerto Rico. They first started with 20 medical students at the University of Puerto Rico. Similar to their previous trials, half of the participants dropped out due to side effects.5 Next, the pair turned to women living in Rio Piedras, a poor neighborhood in San Juan. In total, 265 women were selected to trial their birth control pill. Of these 265 women, 22% of them dropped out due to side effects. Although this was an improvement, the numbers were still significantly high. Further studies were conducted in Puerto Rico, Mexico and Haiti until finally, birth control was approved by the FDA.13
The first clinical trials had similar issues. Women would not continue treatment because of unpleasant side effects. Although the drug was effective, women did not want to experience constant nausea, dizziness, vomiting, headaches and breakthrough bleeding. One reason for such unpleasant side effects was because of the dosage of progestin. On average, patients were receiving 10 mg a day.5 Today, the highest dosage of progestin available in birth control is less than 1 mg. Given the clear difference in dosages, it is no wonder women were not willing to continue treatment.
FDA Approval
G.D. Searle Co. was the first company to manufacture a birth control pill.3 This pill was called Enovid and it received FDA approval in 1957. Although Enovid was FDA approved, the drug could only be used as treatment for infertility and menstrual irregularities.5 It was not until 1959 that Enovid received FDA approval to act as a form of contraception. Following the approval of Enovid, another pharmaceutical company called Syntex began manufacturing their own birth control pill.4 By 1970, seven separate drug companies had received FDA approval to distribute birth control pills and about 9 million American women were using oral contraceptives.2
V. Conclusion
In nine short years, the birth control pill went from an idea requested by Margaret Sanger, to a drug which revolutionized the world both medicinally and socially. Still today, varying methods of reversible contraception are being developed to minimize side effects, maximize efficiency and modify mode of deliverance.
Works Cited
(1) Products – Data Briefs – Number 327 – December 2018. https://www.cdc.gov/nchs/products/databriefs/db327.htm (accessed Apr 5, 2020).
(2) Watkins, E. S. How the Pill Became a Lifestyle Drug: The Pharmaceutical Industry and Birth Control in the United States Since 1960. Am J Public Health 2012, 102 (8), 1462–1472. https://doi.org/10.2105/AJPH.2012.300706.
(3) Kolb, D. A Pill for Birth Control. J. Chem. Educ. 1978, 55 (9), 591. https://doi.org/10.1021/ed055p591.
(4) Madauss, K. P.; Stewart, E. L.; Williams, S. P. The Evolution of Progesterone Receptor Ligands. Med. Res. Rev. 2007, 27 (3), 374–400. https://doi.org/10.1002/med.20083.
(5) Wolfe, A. J. The Partnership Behind The Pill. 2014, 2.
(6) Gebel Berg, E. The Chemistry of the Pill. ACS Cent Sci 2015, 1 (1), 5–7. https://doi.org/10.1021/acscentsci.5b00066.
(7) Pincus, G. The Control of fertility: Gregory Pincus; London, Academic Press: New York, 1968.
(8) Popat, V.; Prodanov, T.; Calis, K.; Nelson, L. The Menstrual Cycle A Biological Marker of General Health in Adolescents. Annals of the New York Academy of Sciences 2008, 1135, 43–51. https://doi.org/10.1196/annals.1429.040.
(9) Lehmann, P. A.; Bolivar A. G.; Quintero, R. R. Russell E. Marker Pioneer of the Mexican steroid industry. Journal of Chemical Education 1973, 50 (3), 195–199. https://doi.org/10.1021/ed050p195.
(10) Djerassi, C. This Man’s Pill: Reflections on the 50th Birthday of the Pill; Oxford UniversityPress: Oxford, 2003.
(11) Djerassi, C. Chemical Birth of the Pill. American Journal of Obstetrics and Gynecology 2006, 194 (1), 290–298. https://doi.org/10.1016/j.ajog.2005.06.010.
(12) Miramontes, L.; Rosenkranz, G.; Djerassi, C. STEROIDS. XXII. THE SYNTHESIS OF 19-NORPROGESTERONE. J. Am. Chem. Soc. 1951, 73 (7), 3540–3541. https://doi.org/10.1021/ja01151a547.
(13) The Bitter Pill: Harvard and the Dark History of Birth Control. Magazine: The Harvard Crimson. https://www.thecrimson.com/article/2017/9/28/the-bitter-pill/ (accessed Mar 1, 2020).